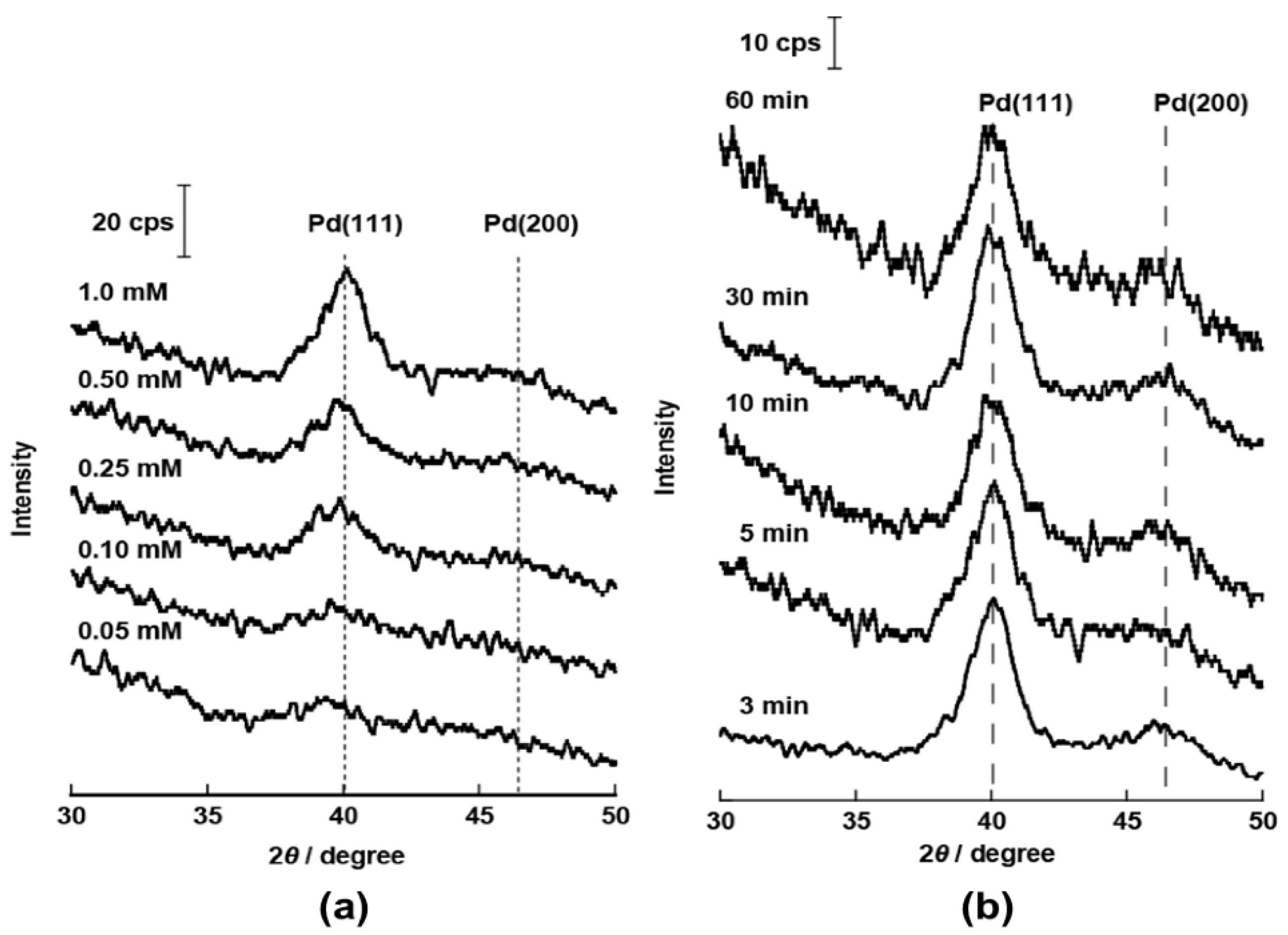
Catalysts | Free Full-Text | Simple Preparation of Pd Core Nanoparticles for Pd Core/Pt Shell Catalyst and Evaluation of Activity and Durability for Oxygen Reduction Reaction

Palladium-Catalyzed C−P Bond Formation: Mechanistic Studies on the Ligand Substitution and the Reductive Elimination. An Intramolecular Catalysis by the Acetate Group in PdII Complexes | Organometallics

The Palladium Acetate‐Catalyzed Microwave‐Assisted Hirao Reaction without an Added Phosphorus Ligand as a “Green” Protocol: A Quantum Chemical Study on the Mechanism - Keglevich - 2017 - Advanced Synthesis & Catalysis -
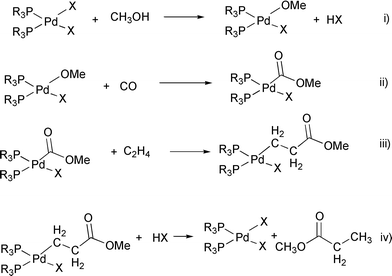
Evidence for the hydride mechanism in the methoxycarbonylation of ethene catalysed by palladium–triphenylphosphine complexes - Journal of the Chemical Society, Dalton Transactions (RSC Publishing) DOI:10.1039/B005232I
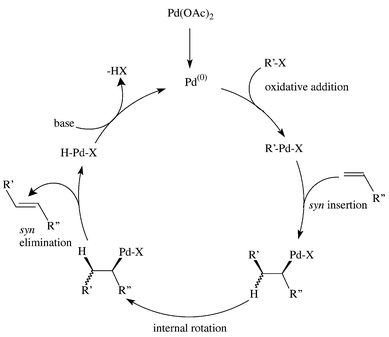
Structural characterisation of solution species implicated in the palladium -catalysed Heck reaction by Pd K-edge X-ray absorption spectroscopy : pall ... - Journal of the Chemical Society, Dalton Transactions (RSC Publishing) DOI:10.1039/B200617K

Understanding Palladium Acetate from a User Perspective - Carole - 2016 - Chemistry – A European Journal - Wiley Online Library

Can Donor Ligands Make Pd(OAc)2 a Stronger Oxidant? Access to Elusive Palladium(II) Reduction Potentials and Effects of Ancillary Ligands via Palladium(II)/Hydroquinone Redox Equilibria | Journal of the American Chemical Society
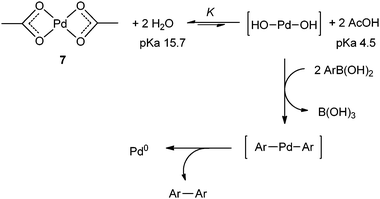
Speciation of Pd(OAc) 2 in ligandless Suzuki–Miyaura reactions - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/C1CY00241D
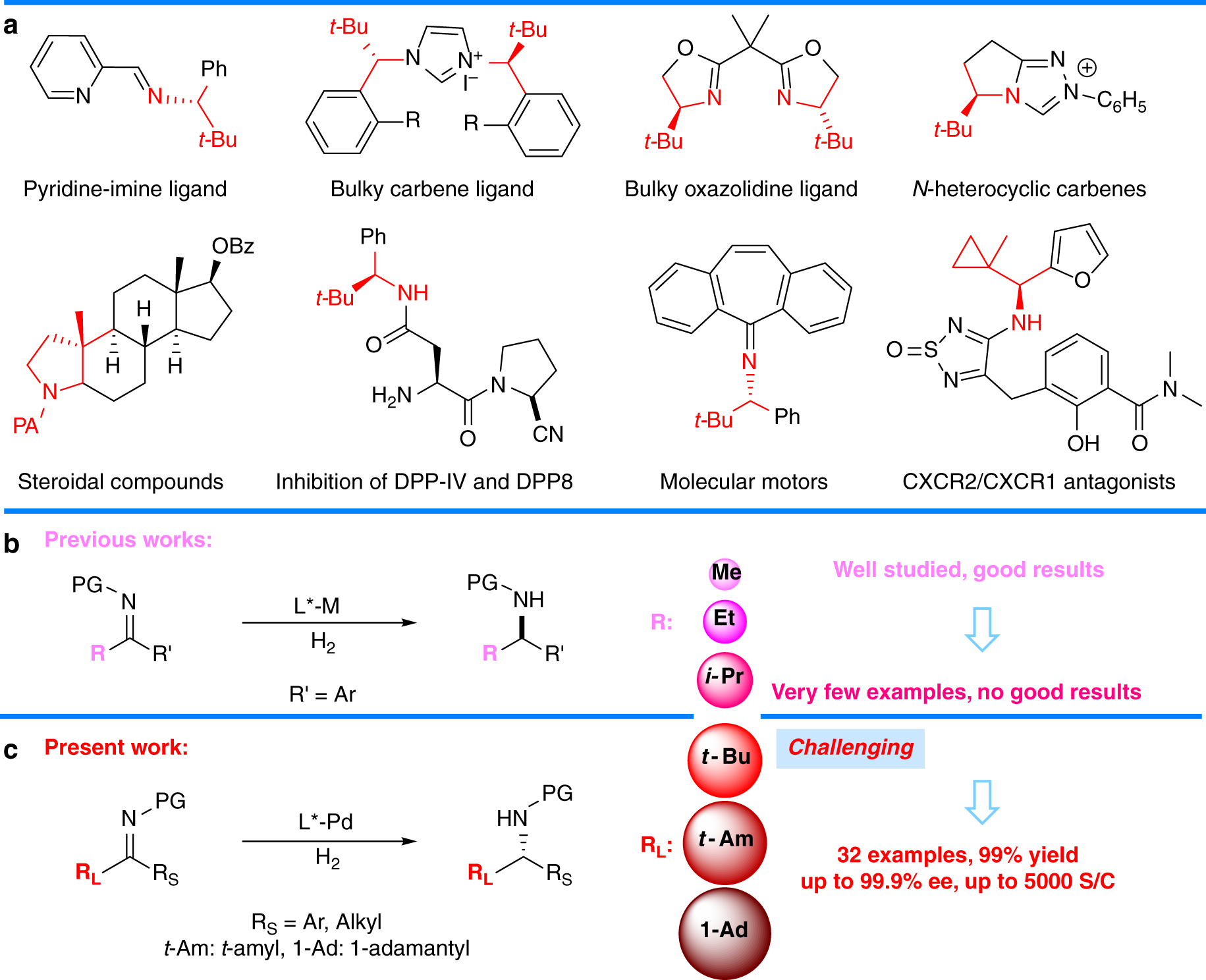
Pd(OAc)2-catalyzed asymmetric hydrogenation of sterically hindered N-tosylimines | Nature Communications

Understanding Palladium Acetate from a User Perspective - Carole - 2016 - Chemistry – A European Journal - Wiley Online Library

Palladium-Catalyzed C−P Bond Formation: Mechanistic Studies on the Ligand Substitution and the Reductive Elimination. An Intramolecular Catalysis by the Acetate Group in PdII Complexes | Organometallics

Formation of Palladium(0) Complexes from Pd(OAc)2 and a Bidentate Phosphine Ligand (dppp) and Their Reactivity in Oxidative Addition | Organometallics

Solvent-Induced Reduction of Palladium-Aryls, a Potential Interference in Pd Catalysis | Organometallics
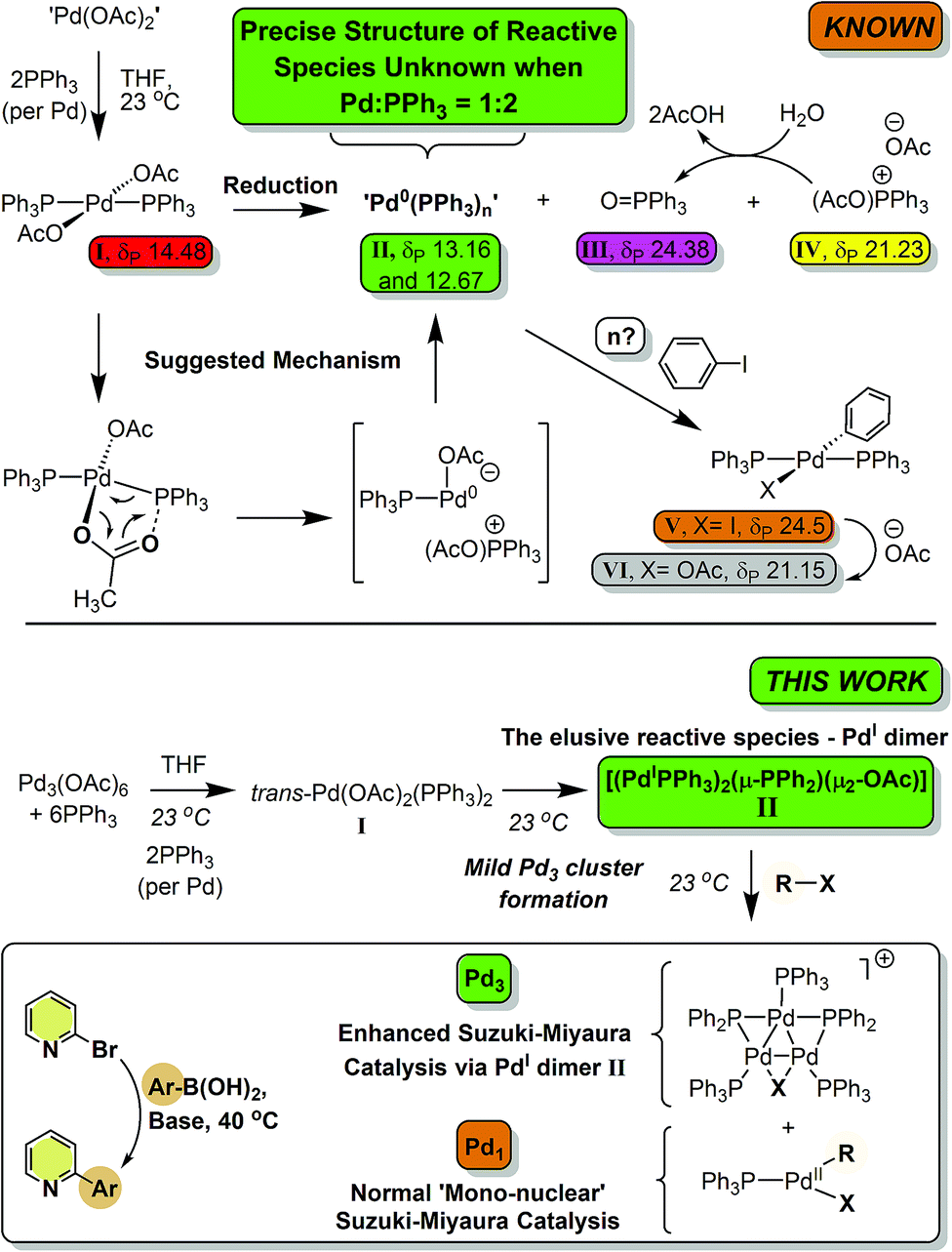
The ubiquitous cross-coupling catalyst system 'Pd(OAc) 2 '/2PPh 3 forms a unique dinuclear Pd I complex: an important entry point into catalytically c ... - Chemical Science (RSC Publishing) DOI:10.1039/C9SC01847F